Self developed biodegradable stent for the first time
Release time:2020-09-01 00:11
On May 30, the launch conference of xinsorb Xinxiang absorbable coronary stent was successfully held at the 14th Oriental cardiology Conference (OCC 2020).
The opening ceremony was officially opened in the welcome speech by academician Ge Junbo, chairman of Zhongshan Hospital Affiliated to Fudan University and OCC conference. Academician Ge Junbo first expressed his gratitude to all online experts for their support to OCC cloud Oriental. As of the end of this conference, the number of participants was 210000. Academician Ge said that on March 4 this year, xinsorb stent was approved and listed by the State Drug Administration, which is the first rapamycin eluting bioabsorbable stent with China's independent intellectual property rights. It is a milestone in the history of stent technology development. Compared with the current DES, the launch of xinsorb will lead the drug-eluting stent Innovation.

Experts participating in xinsorb cloud Oriental listing meeting
Under the leadership of academician Ge Junbo, the experts attending the meeting jointly announced the listing of xinsorb in China, implying that rapamycin, a bioabsorbable coronary artery stent system, has officially embarked on a brilliant journey.
Professor Huo Yong of Peking University First Hospital, Professor Wang Jian'an of the Second Affiliated Hospital of Zhejiang University Medical College, and Professor Wang Haichang of Xi'an International Medical Center served as the chairman of the listing conference. Professor Chen yundai of the General Hospital of the Chinese people's Liberation Army and Professor Zhou Yujie of Beijing Anzhen Hospital served as the chair of the meeting. Professor Hong Lang of Jiangxi Provincial People's Hospital and Jiang of people's Hospital of Wuhan University served as the chairman of the conference Professor Hong, Professor Sun Yingxian of the First Affiliated Hospital of China Medical University, Jiancheng professor of Southern Hospital of Southern Medical University, Professor Yuan Haitao of Shandong Provincial Hospital, Professor Zhang Zheng of the first hospital of Lanzhou university served as the discussion guests, academician Ge Junbo of Zhongshan Hospital Affiliated to Fudan University, Professor Qian Juying of Zhongshan Hospital Affiliated to Fudan University, Professor Shen Li of Zhongshan Hospital Affiliated to Fudan University, and Prof Professor Dong Liang of the Second Affiliated Hospital of Zhejiang University Medical College, as the speaker, jointly demonstrated the research and development process and outstanding performance of xinsorb stent with the mode of new online listing conference.

Recalling the glorious past: academician Ge Junbo reviews the research and development process of xinsorb
In 2005, xinsorb obtained the invention patent - preparation method of biodegradable drug loaded polymer scaffold; from September 2013 to January 2014, we completed the clinical study of FIM; from August 2014 to August 2015, we completed the randomized controlled clinical study; from August 2015 to may 2016, we completed the single group clinical study; in August 2018, we completed the five-year follow-up of the first patient implanted; in March 2020, we completed the follow-up of the first patient In June, xinsorb bioabsorbable stent was approved for marketing.
The research and development project of xinsorb has attracted much attention in the project approval reserve. Premier Li Keqiang also showed great concern at the national outstanding youth project achievement exhibition. The xinsorb project has also won many honors, stepped onto a number of international stages and won a number of national project support.



Professor Qian Juying explained in detail the follow-up data of 4-year randomized controlled clinical trial + 3-year single group registration of xinsorb bioabsorbable stent
The excellent performance of xinsorb has been confirmed in 4-year randomized controlled clinical trials. The primary end point was late stent loss measured by quantitative coronary angiography (QCA) at 12 months postoperatively. The primary end point was that the xinsorb group was not inferior to the Tivoli group (xinsorb 0.19 ± 0.32 mm, Tivoli 0.31 ± 0.42 mm, P non inferiority = 0.003). One year results showed that the xinsorb group had a lower target lesion failure rate (TLF) (Xinsorb 2.6%,TIVOLI 5.3%)。
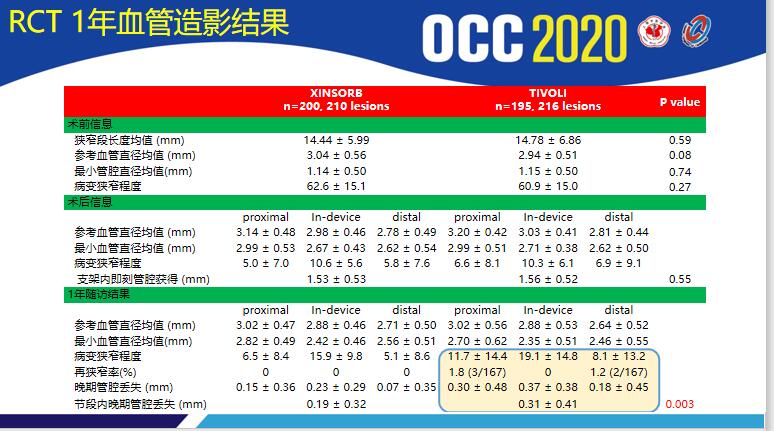
The appearance of xinsorb stent has brought better vascular healing effect for patients, and has become a reliable tool for medical workers in the field of cardiovascular intervention. Single group registration studies showed that xinsorb showed more excellent clinical performance under the guidance of strict indications, reasonable management of dapt and PSP operation principles.

Professor Dong Liang explained in detail the results of 3-year follow-up of randomized controlled + single group clinical trial in the Second Affiliated Hospital of Zhejiang University Medical College
Professor Dong Liang shared the results of a three-year follow-up in the Second Affiliated Hospital of Zhejiang University Medical College. In randomized controlled clinical trials, the primary endpoint was late intrasegmental lumen loss at 12 months after surgery, xinsorb & nbsp; 0.23 ± 0.29, SES 0.36 ± 0.48. In the 3-year follow-up results of randomized trial group and single group registered clinical trial, the secondary efficacy end point was that xinsorb was not inferior to SES (rapamycin eluting stent) at 3 years after operation. Professor Dong Liang said that younger patients with OCT / IVUS follow-up results were more suitable for xinsorb absorbable stent implantation in younger patients, patients with faster absorption and patients with positive remodeling.

15 years of exploration: Professor Shen Li shares xinsorb implantation cases
Professor Shen Li from Zhongshan Hospital Affiliated to Fudan University shared the case of xinsorb implantation. Xinsorb stent system is an absorbable stent system, which has the following characteristics: ① bioabsorbable, and finally achieve no vascular implantation; ② good healing: in the real world, the results of OCT follow-up of patients with xinsorb stent from 6 months to 12 months show that xinsorb has very good endothelial healing performance. ③ Get rid of long-term exposure to polymer and metal scaffolds: biodegradable scaffolds and coatings have no effect on endothelial cell adhesion, so the healing effect is better. The presence of biodegradable stents increases the safety potential without the risk of chronic inflammation and late stent thrombosis. As a result, xinsorb has comparable effectiveness to des; and there are no safety concerns associated with its novel scaffold design and biodegradable materials.
Expert summary
At the end of the launch conference, Professor Huo Yong, Professor Wang Jian'an, Professor Wang Haichang, Professor Chen yundai, Professor Zhou Yujie, Professor Hong Lang, Professor Jiang Hong, Professor Sun Yingxian, Professor Jiancheng, Professor Yuanhai Tao and Professor Zhang Zheng summarized the development advantages of xinsorb absorbable stent, and shared their understanding and expectation of xinsorb stent. Experts all said that the launch of xinsorb stent will bring more selectivity and curative effect to patients. Its unique design concept and production process make xinsorb absorbable stent different from metal drug-eluting stent in vascular healing performance. Professor Huo Yong also sent a message that the listing of xinsorb absorbable stent represents the innovation of medical devices in China, which will add a new choice for Chinese patients receiving interventional therapy. In the future, as Chinese medical workers, we should develop more products with Chinese independent intellectual property rights, and firmly follow the road of Chinese medical device autonomy.
In the future, xinsorb will continue to accompany the Chinese coronary cause and bring better clinical benefits to more Chinese patients.


